Azatadina
Azatadina (nome comercial: Optimina) é um anti-histamínico de primeira geração e anticolinérgico que foi lançado pela Schering-Plough em 1973.[1][2]
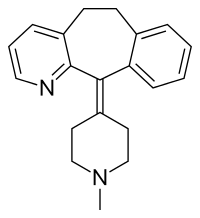 | |
Azatadina | |
| Nome IUPAC (sistemática) | |
| 11-(1-metilpiperidin-4-ildeno)-6,11-di-hidro-5H-benzo[5,6]ciclohepta[1,2-b]piridina | |
| Identificadores | |
| CAS | 3964-81-6 |
| ATC | R06AX09 |
| PubChem | 19861 |
| DrugBank | DB00719 |
| ChemSpider | |
| Informação química | |
| Fórmula molecular | C20H22N2 |
| Massa molar | 290.402 g/mol |
| SMILES | n4c3\C(=C1/CCN(C)CC1)c2ccccc2CCc3ccc |
| Farmacocinética | |
| Biodisponibilidade | ? |
| Metabolismo | ? |
| Meia-vida | ? |
| Excreção | ? |
| Considerações terapêuticas | |
| Administração | ? |
| DL50 | ? |
Foi patenteado em 1967.[3] Tem sido sucedido por outros anti-histamínicos[4] e as aprovações de marketing foram amplamente retiradas.[5][6][7][8][9]
Ver também
Referências
- Katelaris, C. (1990). «Comparative effects of loratadine and azatadine in the treatment of seasonal allergic rhinitis». Asian Pacific Journal of Allergy and Immunology. 8 (2): 103–107. PMID 1982614
- Small, P.; Barrett, D.; Biskin, N. (1990). «Effects of azatadine, terfenadine, and astemizole on allergen-induced nasal provocation». Annals of Allergy. 64 (2 Pt 1): 129–131. PMID 1968324
- Azatadine, Villani, F. J.; Caldwell, W. Patente E.U.A. 3 326 924 (1967).
- Horak F. Antialergic and Vasoactive Drugs for Allergic Rhinitis. Chapter 4 in Allergy Frontiers:Therapy and Prevention. Volume 5 of Allergy Frontiers. Eds Pawankar R et al Springer Science & Business Media, 2010 ISBN 9784431993629
- Drugs.com Drugs.com listing for Azatadine Page accessed July 3, 2015
- Federal Register 2005 Food and Drug Administration Docket No.2005N-0058: Hospira, Inc. et al.; Withdrawal of Approval of 76 New Drug Applications and 60 Abbreviated New Drug Applications 70 FR 10651
- Federal Register 2007 Food and Drug Administration Docket No. 2004P-0262: Withdrawal of Approval of 128 Suitability Petitions 72 FR 8184
- Department of Economic and Social Affairs of the United Nations Secretariat Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or not Approved by Governments Twelfth Issue: Pharmaceuticals United Nations – New York, 2005
- FDA OGD Suitability Tracking Report (Sorted by Drug Name) Page accessed July 3, 2015
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.